Inside The FOXG1 Gene Therapy Program
We’re developing the FOXG1 AAV9 gene replacement therapy—the first-ever treatment designed for FOXG1 syndrome.
The FDA has cleared the clinical trial to begin!
Learn everything about this historic parent-led program and how you can help us reach our $22 million goal to bring this gene therapy to children worldwide.
FRF-001 Clinical Trial Resources
As the FOXG1 gene replacement therapy clinical trial begins, we know parents and caregivers will have many questions.
We will soon be launching a dedicated FRF-001 clinical trial website with the the most up-to-date information and FAQs related to the clinical trial.
You can also find official trial details on ClinicalTrials.gov
What is the FOXG1 Gene Therapy?
Gene therapy is a cutting-edge approach that targets the root cause of genetic disorders by replacing or correcting a faulty gene.
In FOXG1 syndrome, a single gene mutation disrupts brain development and function. Our gene replacement therapy aims to restore healthy FOXG1 expression by delivering a working copy of the gene directly to brain cells.
To do this, we use an AAV9 viral vector—a trusted and widely used delivery system for gene therapy in the brain. Think of the vector as a boat, carrying the healthy FOXG1 gene (the passenger) to the cells that need it. Once delivered, the goal is to replace the loss of FOXG1 protein caused by the mutation, helping the cells function more normally.
Why the Gene Therapy Approach for FOXG1 Syndrome?
FOXG1 syndrome is caused by a mutation in just one copy of the FOXG1 gene. That means the body still has one working copy—it simply needs more of the correct FOXG1 protein. Gene therapy is uniquely suited to this type of disorder because it can deliver a working copy of the gene directly to the brain, increasing FOXG1 protein levels and potentially improving key neurological functions. Current scientific data and preclinical models give us real reason to believe that gene therapy can make a meaningful difference in the lives of children with FOXG1. That’s why we’re moving urgently—and carefully—toward and through clinical trials.
Our preclinical data shows that this technique works using various types of AAV vectors (boats) in animal models of FOXG1 syndrome, where we observe treatment-induced increase in expression of FOXG1 in animals’ brains and correspondingly we observe improvement in symptoms in the animals models, which recapitulate some of the symptoms characteristic of human FOXG1 syndrome patients. We found groundbreaking results showing restoration of the brain structural abnormalities (agenesis of the corpus callosum).
FOXG1 Gene Therapy Team
Gai Ayalon, Ph.D.
Chief Drug Development Officer
Dr. Gai Ayalon, Ph.D., is a neuroscientist with a passion for bringing treatments to children with ultra-rare diseases. As Chief Drug Development Officer, Dr. Ayalon is leading the FOXG1 Research Foundation’s gene therapy program through clinical development. He has built and leads the Foundation’s core gene therapy team, which includes Charles River Laboratories and other key partners advancing the program toward clinical trials.
Previously, Dr. Ayalon led clinical development programs for pediatric neurodevelopmental disorders at Ultragenyx Pharmaceutical, where he also launched and piloted clinical readiness teams to accelerate the transition from preclinical research to human trials.
-

Brandon Michael Henry
Chief Medical Officer
-

Mallory Lauth, MS
Founder & CEO, Lauth Life Sciences | Program Management & Clinical Operations Consulting
-

Elli Brimble
FRF Chief Clinical Data Officer
-

Lauren E. Black PhD
Scientific Advisory, Preclinical Strategy, Charles River Laboratories
-

Carol Zoltowski, VMD
Principal of CZ Associates, LLC | Regulatory Affairs & Quality Consulting
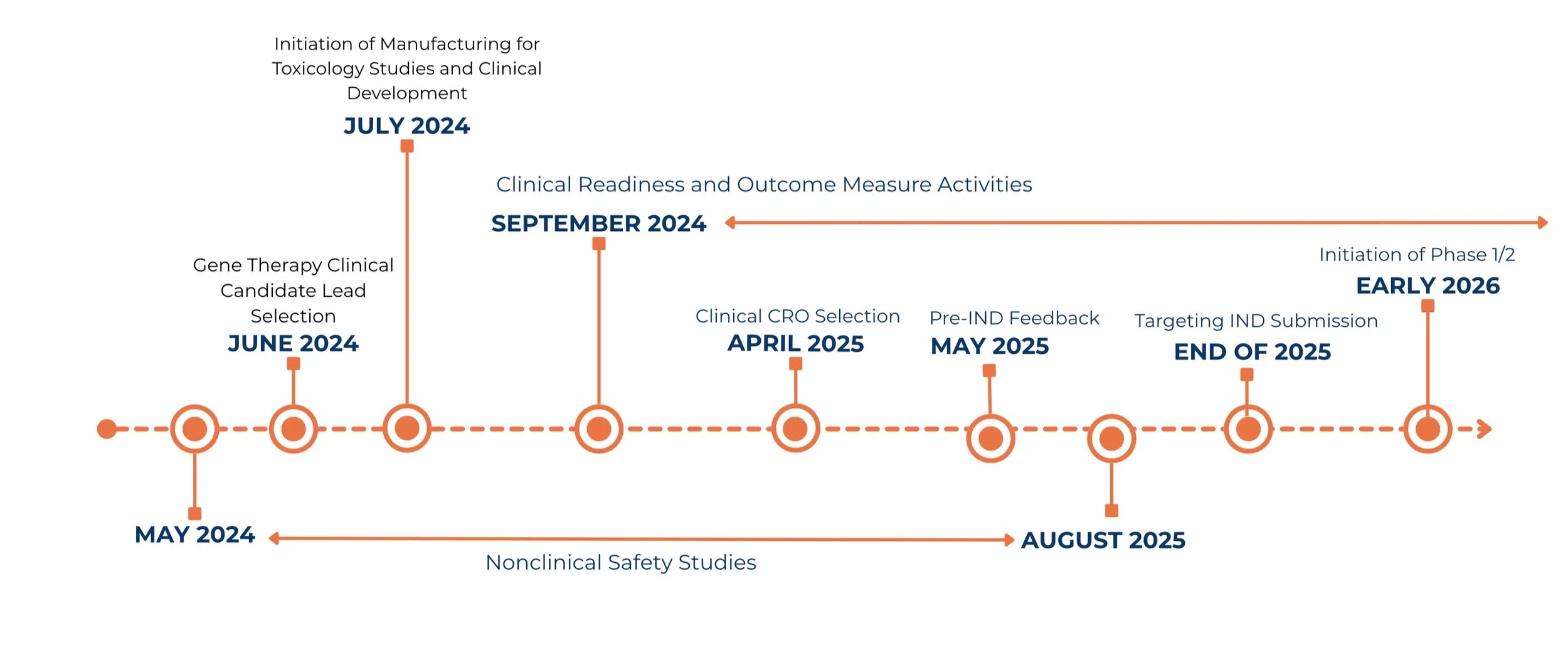
FOXG1 Gene Therapy Roadmap
Help Us Drive the FOXG1 Gene Therapy Through Patient Trials—Starting in 2026.
Our “Yes, They Can!” FOXG1 Gene Therapy campaign is raising $22 million to independently fund the FOXG1 gene therapy through full-scale, multi-site patient trials.

Watch Dr. Gai Ayalon discuss the FOXG1 gene therapy
At the 2024 FOXG1 Syndrome Parents Conference in Florida, Dr. Gai Ayalon presented on the roadmap to deliver the FOXG1 gene therapy to patients around the world. Watch Gai’s presentation here.