2025 Impact Report : FOXG1 Research Foundation
2025 marked eight years of the FOXG1 Research Foundation, a global, parent-driven organization dedicated to improving the lives of everyone affected by FOXG1 syndrome.
This was our most pivotal year yet—we successfully completed toxicology studies and submitted an Investigational New Drug (IND) application to the FDA to begin the first-ever FOXG1 gene therapy clinical trial in 2026. Every decision, every dollar raised, and every milestone achieved in 2025 was made with one clear focus: responsibly and urgently preparing to treat children through patient clinical trials.
In parallel, we continued writing a new playbook for rare disease drug development— demonstrating that a parent-led foundation can and will independently sponsor and execute a multi-site, international clinical trial. This work is not only transforming what is possible for FOXG1 syndrome, but also creating a replicable blueprint for other rare disease communities facing the same unmet need.
As we moved meticulously along the gene therapy roadmap, 2025 also brought remarkable progress across every pillar of our mission. We achieved major breakthroughs in patient continuity of care with the establishment of a dedicated ICD-10 code, published groundbreaking findings from our Natural History Study offering the most comprehensive understanding of FOXG1 syndrome to date, secured key FDA designations for our gene therapy program, expanded our global patient and supporter community, and mobilized unprecedented awareness through initiatives such as Tom Horton’s 500-mile walk and a FOXG1 family featured on ESPN, and amplified by Tom Brady.
While we made historic progress in 2025, it was also a year marked by deep sorrow. Our community lost eight beautiful individuals with FOXG1 syndrome. We honor their lives and stand with their families, carrying their memory forward as a constant reminder of why this work cannot wait.
None of our progress would be possible without our donors, families, scientific partners, clinicians, and volunteers. Together, we are proving what parent-led drug development can achieve.
Key Accomplishments
FOXG1 Research Fundraising
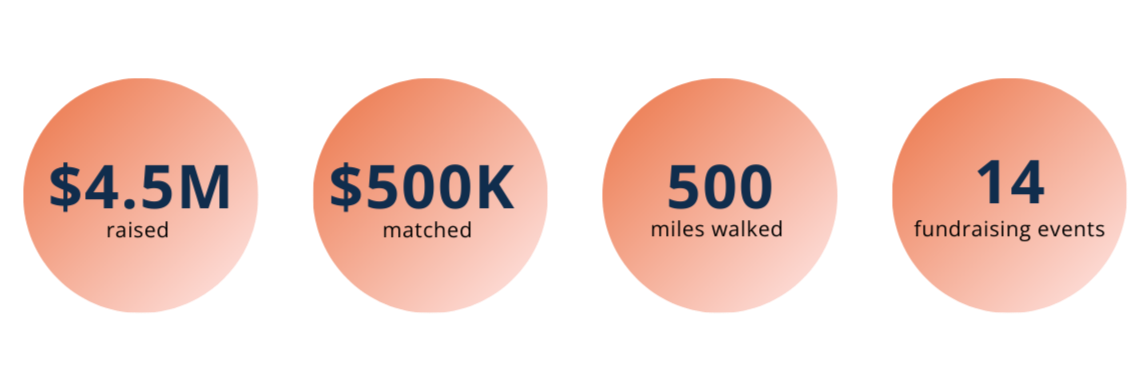
• Raised $4.5 million in 2025, reaching $14.5 million toward our $22 million “Yes, They Can!” campaign, directly advancing the FOXG1 gene replacement therapy program toward patient clinical trials
• Surpassed our peer-to-peer Frankie $500K end-of-year goal of $500,000 and doubled the impact to $1 million, made possible by a $500,000 matching commitment from Fondation Thot
• Tom Horton’s 500-mile walk across El Camino de Santiago raised $2 million and generated global awareness for FOXG1 syndrome and parent-led drug development
• Continued to grow a diversified global donor base committed to funding rare disease drug development and improving lives for rare disease families
FOXG1 Drug Development
• Completed IND-enabling toxicology studies
• Completed clinical-grade manufacturing of the gene therapy product
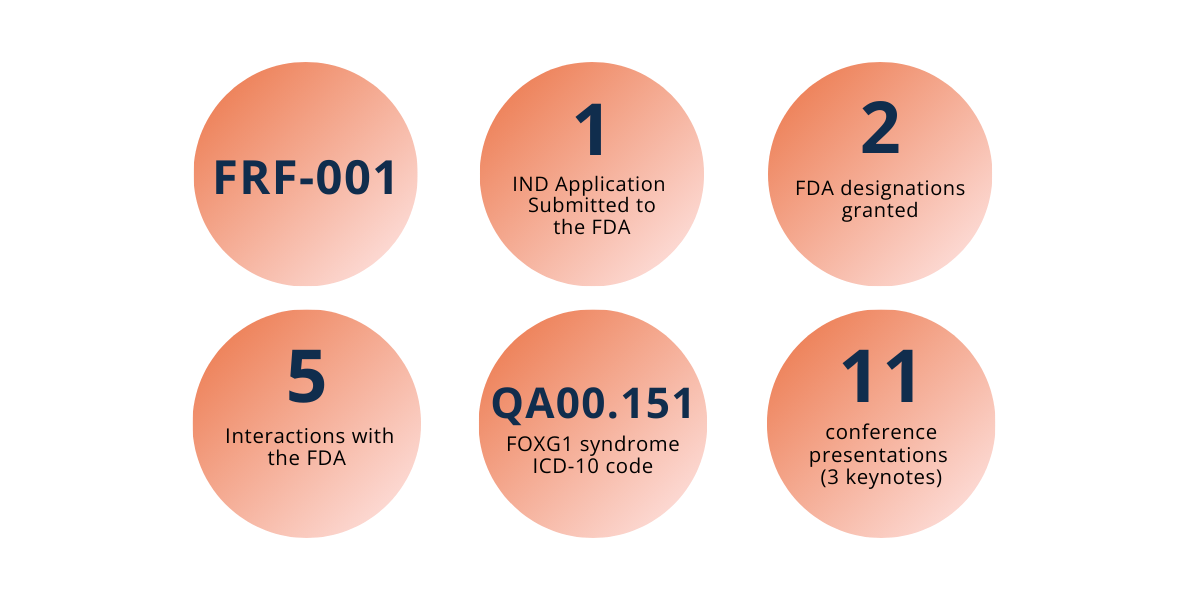
• Conducted a successful pre-IND interaction with the FDA earlier in the year, incorporating regulatory guidance into final trial preparations
• Conducted two parent and caregiver advisory board meetings to obtain input from FOXG1 families on logistical aspects of clinical trial design and conduct
• Conducted advisory board meeting with key pediatric neurologists to finalize the clinical trial design
• Achieved multiple key FDA designations, including Orphan Drug Designation and Rare Pediatric Disease Designation, strengthening regulatory alignment and accelerating the path to clinical trials
• Submitted the Investigational New Drug (IND) application for FRF-001 to the FDA in December 2025
• Listed FRF-001 on ClinicalTrials.gov
• Submitted a Fast Track Designation application to the FDA for FRF-001
• Expanded partnerships and collaborations with leading vendors to support upcoming clinical trial execution and data integrity, including a key partnership with IQVIA Biotech as our clinical trial vendor
• Further established a new model for independent, parent-led rare disease drug development—demonstrating that life-changing therapies can be advanced faster and at a fraction of traditional costs
• Published new paper from the FOXG1 Research Center on insights into the pathophysiology of FOXG1 syndrome
• Continued research efforts beyond AAV9 gene therapy, including antisense oligonucleotide work supported through the FOXG1 Research Center and broader scientific collaborations to deepen understanding of FOXG1 biology and disease progression
FOXG1 Clinical Advancements and Patient Support
• Secured a unique ICD-10 code for FOXG1 syndrome, formally recognizing FOXG1 as a distinct diagnosis in global medical systems and improving patient continuity of care
• Published findings from the FOXG1 Natural History Study, offering the most comprehensive clinical picture of FOXG1 syndrome to date and directly informing trial design
• Introduced an AI-powered Advocate for FOXG1 parents and caregivers through our partnership with Citizen Health, providing real-time support and access to medical records
• Identified 508 new FOXG1 patients worldwide, expanding access to research, resources, and community
• Validated 79 new patients to the official FOXG1 Patient Registry and added 40 new patients to the Citizen Health FOXG1 Natural History Study
FOXG1 Advocacy, Awareness, & Global Growth
• Actively engaged in policy advocacy to protect the rights of individuals with disabilities and to protect and advance incentives for rare disease research, ensuring life-changing and life-saving treatments can be developed for populations with the greatest unmet medical need
• Continued to grow and mobilize our global FOXG1 volunteer and parent leadership network
• Added four new FOXG1 Research Foundation affiliate chapters added in UK, Mexico, Russia, and Japan
• Presented Keynote speeches, and attended major scientific and medical conferences, including:
– American Society of Gene and Cell Therapy Annual Conference (ASGCT)
– American Epilepsy Society Annual Conference (AES)
– Syneos SyncUp Summit
– Epilepsy Awareness Day at Disneyland
– Advanced Therapies Dallas
– SCOPE Summit (Keynote Speaker)
– MATTER Advancing Brain Health Summit (Keynote Speaker)
– Dup15q Family Conference (Keynote Speaker)
– Inclusion Done Right Conference, Stony Brook University (Keynote Speaker)
– BIO International Convention
– Charles River Rare Disease Summit
• Elevated FOXG1 awareness through national and international media, including an ESPN feature highlighting the Luckie family, which reached a global audience with amplification by Tom Brady!
Looking Ahead: 2026 Goals - The Year of the first FOXG1 Treatment!
FOXG1 Gene Therapy Program
• Launch FRF-001 clinical trial and initiate enrollment
• Engage ex-U.S. regulatory agencies to expand the trial internationally
FOXG1 Fundraising and Global Growth
• Raise the remaining $7.5 million to complete the “Yes, They Can!” campaign to advance the FOXG1 gene therapy program through patient clinical trials
• Host fundraising and awareness events worldwide, including an opera in Paris
• Convene a FOXG1 Research Foundation Global Leadership Meeting in Paris
• Launch new affiliate FOXG1 Research Foundation chapters globally
FOXG1 Community, Caregiver, and Clinical Support
• Deepen collaboration among families, scientists, clinicians, and regulators
• Expand FOXG1 parent and caregiver support tools, including new AI-enabled resources to assist with daily caregiving and medical navigation, including epilepsy care and FOXG1 standards of care
• Host the 2026 FOXG1 Family Getaway at Morgan’s Wonderland, a fully accessible waterpark in Texas
• Continue the success of our FOXG1 support groups and new parent meetings
Advocacy and Education
• Continue advocating alongside our network of rare disease partners to promote and protect the human rights of individuals with rare diseases and developmental disabilities, supporting their inclusion and full participation in their communities
• Continue engaging with Congressional leaders to support the reauthorization of the Rare Pediatric Disease Priority Review Voucher (PRV) program through the Give Kids a Chance Act—an essential incentive to ensure promising therapies can reach children who urgently need them
• Expand early education and awareness around disability inclusion, rare diseases, and FOXG1 syndrome through children’s books, student assemblies, and educational programs
Thank you to everyone who supports our parent-led organization in any way—through donations, pro bono expertise, volunteering, partnerships, or even by quietly cheering us on from afar. They say it takes a village, and ours is truly global, connected by a shared sense of love, urgency, and determination to create a world where rare diseases are no longer an unmet need. We look forward to our future Impact Reports where we no longer share news of lives lost, but instead celebrate how many lives are improved by the advancements our programs are making possible.